|
Case Report
The forgotten fragment: Unmasking a rare case of calcified retained placenta at 20 weeks postpartum
1 Edward Via College of Osteopathic Medicine, Blacksburg, Virginia, USA
2 Inova Fair Oaks Hospital, Fairfax, Virginia, USA; Tepeyac OB/GYN, Fairfax, Virginia, USA
Address correspondence to:
Yasaman Dasteh Goli
Edward Via College of Osteopathic Medicine, Blacksburg, Virginia,
USA
Message to Corresponding Author
Article ID: 100040G06YG2025
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Dasteh Goli Y, Cvetkovich LL. The forgotten fragment: Unmasking a rare case of calcified retained placenta at 20 weeks postpartum. Edorium J Gynecol Obstet 2025;9(1):1–5.ABSTRACT
Introduction: Retained products of conception (RPOC) are a recognized cause of secondary postpartum hemorrhage, most commonly occurring within the early postpartum period. However, delayed presentations with calcified RPOC are rare and pose unique diagnostic challenges. While advanced maternal age, uterine atony, prior cesarean sections, and abnormal placentation— such as placenta accreta spectrum and succenturiate placenta—are common risk factors, RPOC can also occur in low-risk patients and require careful clinical management.
Case Report: A 25-year-old G1P1 woman with an uncomplicated pregnancy and vaginal delivery presented with persistent intermittent bleeding 20 weeks postpartum. Despite undetectable serum hCG levels and no signs of infection, serial ultrasounds revealed a calcified mass at the uterine fundus. The patient underwent hysteroscopy with dilation and curettage (D&C), confirming the presence of calcified placental fragments with fibrosis. Her symptoms resolved postoperatively, and no complications were observed. The findings suggest a likely retained succenturiate lobe, an uncommon placental abnormality that was undetected in the immediate postpartum period.
Conclusion: This case highlights the importance of clinical vigilance and timely intervention in managing postpartum complications, particularly in an evolving obstetric population where advanced maternal age and the use of assisted reproductive technologies are becoming more prevalent. Early identification of RPOC, aided by imaging modalities such as ultrasound and saline infusion sonohysterography, and prompt treatment with hysteroscopy and D&C are essential to prevent complications such as chronic endometritis and intrauterine adhesions. Recognizing rare placental anomalies is critical for optimizing patient outcomes.
Keywords: Calcified placental tissue, Delayed postpartum bleeding, Postpartum bleeding, Retained products of conception (RPOC), Succenturiate placenta
INTRODUCTION
Retained products of conception (RPOC) refer to placental or fetal tissue remaining in the uterus following delivery. Occurring in approximately 0.5–3% of all vaginal deliveries, RPOC is a significant contributor to secondary postpartum hemorrhage (PPH), defined as excessive vaginal blood loss between 24 hours and 6–12 weeks postpartum [1],[2],[3]. The incidence of secondary PPH typically peaks within one to two weeks postpartum; however, cases extending beyond 12 weeks, though rare, are clinically significant [3],[4] .
Various factors predispose women to RPOC, including uterine atony, advanced maternal age, prior cesarean sections, multiple gestation, prolonged labor, premature delivery, and manual removal of the placenta. Abnormal placentation, such as placenta accreta spectrum (PAS) and succenturiate placenta, presents substantial risks for retained placental tissue and postpartum complications. It is characterized by abnormal adherence of the placenta to the uterine wall, making complete detachment difficult during delivery [1],[2],[3],[4],[5]. Risk factors for PAS include previous cesarean sections, uterine surgeries, maternal age ≥35, and the use of assisted reproductive technologies (ART), all of which can alter uterine architecture, increasing the likelihood of placental retention. Conversely, a succenturiate placenta involves the development of one or more small accessory lobes separate from the main placental body, connected by fetal vessels [6]. Despite sharing advanced maternal age and ART risk factors with PAS, a succenturiate placenta can also arise from implantation over previous surgical sites, uterine anomalies such as leiomyomas, or at the cervical os [6],[7].
While both conditions elevate the risk of RPOC, PPH, and infection, their distinguishing feature lies in the pattern of placental development and detachment. Placenta accreta spectrum results from abnormal adherence, making manual removal difficult, whereas succenturiate placenta tends to leave behind accessory lobes that may not be detected during initial postpartum inspection [5],[6],[7].
Furthermore, increased duration of labor, particularly the first and second stages, is of special concern in nulliparous women. Poor uterine contraction, commonly due to prolonged oxytocin use, high parity, or uterine anomalies further contributes to the risk of placental retention [1],[2],[3],[4],[5]. Despite these known risk factors, RPOC can also develop unexpectedly in patients without predisposing conditions. Retained placental fragments compromise uterine contraction and involution, resulting in localized bleeding or hypoperfusion, while also serving as a nidus for infection and increasing the risk of postpartum complications such as endometritis or sepsis [3],[4],[5],[8].
Grayscale ultrasound with color Doppler is critical for diagnosing RPOC. Findings often include a thickened endometrial lining or an echogenic mass representing retained tissue [9],[10],[11]. In chronic cases, the retained tissue may undergo dystrophic calcification, leading to more subtle clinical symptoms rather than the usual heavy bleeding, cramping, and fever associated with early postpartum RPOC [9],[10],[11],[12]. Saline infusion sonohysterography (SIS) enhances uterine cavity visualization, especially when ultrasound results are inconclusive, by differentiating between active and calcified tissue [8],[13].
Hysteroscopy with dilation and curettage (D&C) is the definitive diagnostic and therapeutic approach, allowing for direct visualization and safe removal of retained tissue. This procedure is particularly effective in cases involving calcified or adherent placental fragments, reducing the risks of complications such as intrauterine adhesions or endometritis. Compared to blind curettage, hysteroscopy lowers the risk of uterine injury and is the preferred option when the spontaneous resolution of RPOC is unlikely [11],[12],[13].
This case report highlights an unusual presentation of RPOC at 20 weeks postpartum, emphasizing the importance of clinical vigilance even in the absence of traditional risk factors or early symptoms. Delayed cases, though uncommon, can lead to significant complications if not properly diagnosed and managed.
CASE REPORT
A 25-year-old G1P1 woman, with no significant past medical or surgical history, presented to the outpatient clinic with continuous postpartum bleeding. She had an uncomplicated singleton pregnancy and normal spontaneous vaginal delivery at 40 weeks and 4 days of gestation. The placenta was delivered promptly after fundal massage, suprapubic pressure, and gentle traction on the umbilical cord. Upon inspection, the placenta appeared complete and intact, with a central three-vessel cord, and her immediate postpartum course was uneventful.
However, after the resolution of normal lochia, the patient reported two weeks of heavy bleeding, followed by what seemed like a normal menstrual period. Subsequently, she experienced intermittent light bleeding for approximately nine weeks postpartum, which tapered off two days before her outpatient visit. Despite these symptoms, the patient reported feeling well with no other complaints.
Over the course of the patient’s follow-up, serial pelvic ultrasounds were performed approximately one month apart revealing:
- A retroverted uterus with unremarkable myometrial echotexture.
- A heterogeneous endometrium, with internal flow, measuring 8 mm initially, reducing to 5 mm on subsequent scans.
Her ovaries consistently showed multiple small peripheral follicles, suggesting polycystic ovarian syndrome (PCOS), with no adnexal masses or free fluid.
Given the persistence of intermittent bleeding and ultrasound findings, chronic endometritis was considered a differential diagnosis. However, since the patient’s symptoms had improved, an informed decision was made to monitor the situation. A short course of antibiotics and SIS for further evaluation were offered in case of a recurrence of bleeding, but the patient declined any immediate intervention. Over the next few weeks, the patient experienced several more days of light brown bleeding, which resolved completely.
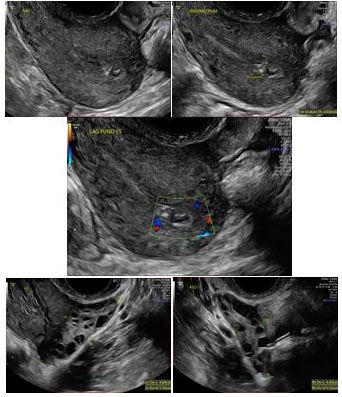
At 19 weeks 5 days postpartum, the patient returned with persistent intermittent bleeding. She reported no associated symptoms such as fever, chills, or abdominal or pelvic pain and did not require medical attention until the bleeding became more concerning. Uterine involution had been normal during previous postpartum visits, and she had not experienced symptoms suggestive of ovulation. Her human chorionic gonadotropin (hCG) serum levels were below detectable limits (<5 mIU/mL). Despite completion of a course of antibiotics, her symptoms persisted, and the latest serial sonographic examination showed an echogenic area of calcification, measuring up to 13 × 7 × 10 mm, at the uterine fundus, raising suspicion for retained placental tissue (Figure 1). The patient was extensively counseled on the need for hysteroscopic D&C given the chronicity of symptoms and the ongoing concern for RPOC, and she agreed to proceed with the procedure.
Surgical procedure
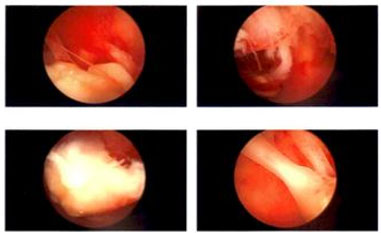
Hysteroscopy revealed a small, white, discreet calcified mass attached to the right lateral uterine wall (Figure 2). Both tubal ostia were visible. A light morcellator was introduced to remove the firm mass, and a sharp curettage was performed. Hemostasis was achieved, and the patient was sent to the recovery room in stable condition. Histopathological examination confirmed proliferative endometrium and the presence of retained placental fragments with significant calcification and fibrosis. No discrete cystic structures or fetal parts were identified. The patient’s postoperative course was uneventful, and she was discharged home with complete resolution of her symptoms. She continued follow-up care without further complications.
DISCUSSION
This case illustrates the rare presentation of RPOC manifesting five months after an otherwise uncomplicated delivery. Retained placental tissue typically exerts hormonal effects early in the postpartum period, causing heavy bleeding, cramping, and an enlarged, boggy uterus. However, in this case, the absence of early symptoms suggested that the tissue had lost its hormonal activity and become biologically inert through dystrophic calcification. The calcified tissue no longer produces hormones like hCG or progesterone, explaining the association with only intermittent bleeding at 20 weeks postpartum. By this time, uterine involution had already occurred, and breastfeeding likely further suppressed ovulation, masking the usual signs of retained placental tissue, such as uterine atony or PPH.
Retained placental tissue often results from abnormal placentation; however, no evidence of PAS was found in this patient’s pathology. Although antenatal ultrasounds in this case did not reveal any concerning placental anomalies, and the placenta appeared intact upon inspection after delivery, the calcified mass discovered 20 weeks later could likely represent a retained succenturiate lobe that was missed during the initial postpartum period.
One key aspect of this case is the apparent decline in hormonal activity of the RPOC, as evidenced by negative serum hCG levels despite the persistence of placental fragments. This pattern aligns with findings from Torrenga et al. [12] which demonstrated that while hormonal activity of placental tissue may diminish relatively quickly, tissue degeneration, as visualized on imaging, can take considerably longer. Here, the placental tissue persisted beyond the resolution of heavy bleeding and normalization of hCG levels, suggesting that hormonal decline may not always correspond with complete tissue resolution. The absence of internal vascularization in calcified tissue, as observed in this case, confirms that the tissue was no longer viable and unlikely to resolve spontaneously.
While conservative management may be appropriate in some cases, especially when the tissue is non-vascularized or the patient is asymptomatic, management must be tailored to the clinical scenario. For calcified RPOC, timely intervention with hysteroscopy and D&C is essential to prevent further complications, such as chronic endometritis or intrauterine adhesions, both of which can significantly impact reproductive health and future fertility outcomes. Therefore, ongoing monitoring with serial ultrasounds is crucial to detect persistent tissue and assess the need for further intervention, especially when surgical management is deferred, or the clinical presentation is atypical.
Hysteroscopy with D&C offers diagnostic and therapeutic advantages. It not only allows for direct visualization of the retained or calcified tissue but also minimizes the risk of uterine injury. Additionally, hysteroscopy provides valuable insights into uterine anatomy and endometrial health, which is critical for advising patients on future pregnancies and reducing the risk of recurrence or abnormal placental adherence. This approach ensures both diagnostic accuracy and effective treatment in a single procedure, thereby reducing the likelihood of further complications.
Ultimately, it is essential to counsel women with a history of RPOC regarding the potential for placental abnormalities in future pregnancies. The evolving characteristics of the obstetric population—including maternal age ≥35, higher prevalence of nulliparity, medicalization of labor, and increased use of ART—have contributed to a greater likelihood of abnormal placentation and other pregnancy-related complications. Given these factors, maintaining close surveillance of women with a history of RPOC is imperative. Serial sonographic monitoring and early localization of the placenta in subsequent pregnancies are essential for identifying any signs of abnormal placental invasion, which may warrant closer follow-up or even consideration for an elective cesarean section. Moreover, patients with RPOC should be closely monitored postpartum, with hysteroscopic valuation recommended at one year to confirm the complete resolution of retained tissue and prevent future complications.
This case underscores the importance of continued clinical vigilance, tailored intervention, and thorough counseling of women with RPOC to ensure optimal management of future pregnancies and reproductive health.
CONCLUSION
This case emphasizes the critical need for clinical vigilance in diagnosing and managing delayed cases of RPOC, even in low-risk postpartum patients. The evolving obstetric population, with increased rates of advanced maternal age, ART use, and nulliparity, heightens the risk of complications like RPOC. Timely intervention, facilitated by imaging and hysteroscopic evaluation, is essential to prevent complications such as chronic endometritis and intrauterine adhesions. Comprehensive postpartum follow-up, including counseling about potential future placental abnormalities, ensures optimal reproductive outcomes for affected patients.
REFERENCES
1.
Perlman NC, Carusi DA. Retained placenta after vaginal delivery: Risk factors and management. Int J Womens Health 2019;11:527–34. [CrossRef]
[Pubmed]

2.
Coviello EM, Grantz KL, Huang CC, Kelly TE, Landy HJ. Risk factors for retained placenta. Am J Obstet Gynecol 2015;213(6):864.e1–11. [CrossRef]
[Pubmed]

3.
Favilli A, Tosto V, Ceccobelli M, et al. Risk factors for non-adherent retained placenta after vaginal delivery: A systematic review. BMC Pregnancy Childbirth 2021;21(1):268. [CrossRef]
[Pubmed]

4.
Shinohara S, Okuda Y, Hirata S, Suzuki K. Predictive factors for secondary postpartum hemorrhage: A case-control study in Japan. J Matern Fetal Neonatal Med 2022;35(20):3943–7. [CrossRef]
[Pubmed]

5.
Tchuinte Lekuikeu LS, Moreland C. Retained placenta and postpartum hemorrhage: A case report and review of literature. Cureus 2022;14(4):e24389. [CrossRef]
[Pubmed]

6.
Kellow ZS, Feldstein VA. Ultrasound of the placenta and umbilical cord: A review. Ultrasound Q 2011;27(3):187–97. [CrossRef]
[Pubmed]

7.
Suzuki S, Igarashi M. Clinical significance of pregnancies with succenturiate lobes of placenta. Arch Gynecol Obstet 2008;277(4):299–301. [CrossRef]
[Pubmed]

8.
Clemente-Tomás CP, Abril-Utrillas N, Cebolla-Gil P, et al. Alternative management of placenta accreta: A case report. Acta Scientific Medical Sciences 2023;7(8):112–4. [CrossRef]

9.
De Winter J, De Raedemaecker H, Muys J, Jacquemyn Y. The value of postpartum ultrasound for the diagnosis of retained products of conception: A systematic review. Facts Views Vis Obgyn 2017;9(4):207–16.
[Pubmed]

10.
Mulic-Lutvica A, Axelsson O. Ultrasound finding of an echogenic mass in women with secondary postpartum hemorrhage is associated with retained placental tissue. Ultrasound Obstet Gynecol 2006;28(3):312–9. [CrossRef]
[Pubmed]

11.
Velebil P, Hympanova LH, Herman H, Emingr M, Krepelka P, Hanacek J. Retained products of conception – A retrospective analysis of 200 cases of surgical procedures for the diagnosis of residua postpartum. Ginekol Pol 2023;94(12):967–71. [CrossRef]
[Pubmed]

12.
Torrenga B, Huirne JA, Bolte AC, van Waesberghe JHTM, de Vries JIP. Postpartum monitoring of retained placenta. Two cases of abnormally adherent placenta. Acta Obstet Gynecol Scand 2013;92(4):472–5. [CrossRef]
[Pubmed]

13.
Guarino A, Di Benedetto L, Assorgi C, Rocca A, Caserta D. Conservative and timely treatment in retained products of conception: A case report of placenta accreta ritention. Int J Clin Exp Pathol 2015;8(10):13625–9.
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Yasaman Dasteh Goli - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Lorna L Cvetkovich - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2025 Yasaman Dasteh Goli et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.