|
Case Report
Male breast cancer: Case report and review of literature
1 Mohamed VI Center of Oncology and Gynecology, Obstetrics and Gynecology Department, University Hospital Ibn Rochd, Faculty of Medicine and Pharmacy, Hassan II University of Casablanca, Casablanca, Morocco
Address correspondence to:
Amal Cherkaoui
Mohamed VI Center of Oncology and Gynecology, Obstetrics and Gynecology Department, University Hospital Ibn Rochd, Faculty of Medicine and Pharmacy, Hassan II University of Casablanca, Casablanca,
Morocco
Message to Corresponding Author
Article ID: 100038G06AC2024
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Cherkaoui A, Nouri M, Tossi S, Armel H, Bencherifi Y, Benhassou M, Ennachit S, El Karroumi M. Male breast cancer: Case report and review of literature. Edorium J Gynecol Obstet 2024;9(2):5–9.ABSTRACT
Introduction: Male breast cancer (MBC) is an uncommon disease that makes up less than 1% of all breast cancer diagnoses worldwide. While breast carcinomas have some similarities in both sexes, there are significant differences.
Case Report: We present a case of breast cancer in a 57-year-old man who had a left breast mass of 3.5 cm classified as breast imaging reporting and data system (BIRADS 4) by the American College of Radiology (ACR). The presence of invasive breast carcinoma of grade II Scarff–Bloom–Richardson (SBR) with a negative molecular phenotype B HER2 was confirmed by a tru-cut biopsy. The axillary cytopuncture did not show any abnormalities. The patient was later treated for a left mastectomy with homolateral axillary dissection.
Conclusion: Male breast cancer represents a therapeutic dilemma. A multidisciplinary approach is mandatory.
Keywords: Breast cancer, Mastectomy, Oncology
INTRODUCTION
Breast cancer in men is rare, accounting for only about 1% of all breast cancers and less than 1% of all male neoplasia [1]. The risk of developing another cancer is higher for men who have had breast cancer. Breast cancer metastases are mostly found in the bones, representing about 62% of cases [2],[3].
The causes of breast cancer in men remain largely unknown according to various documentary sources [3],[4]. However, the risk factors identified include family history of breast cancer, a genetic predisposition affecting about 15% of cases of male breast cancer and associated with a genetic mutation inherited from the BRCA2 gene [5], radiation exposure, particularly in the thorax, as well as liver cirrhosis, which are also documented risk factors. Cirrhosis is suspected of increasing estrogen levels and decreasing androgen levels [1],[6]. Other risk factors include gynecomastia, obesity, excessive alcohol consumption, excessive smoking, and environmental factors [1],[7].
The most common symptom in breast cancer in men is pain, which often occurs around a mass that appears gradually [6]. The most common carcinoma in humans remains infiltrating ductal carcinoma, while other types of cancers are very rare [8],[9]. Symptoms and management of breast cancer in men are similar to those observed in women, as treatment generally follows the same regimen [10],[11].
CASE REPORT
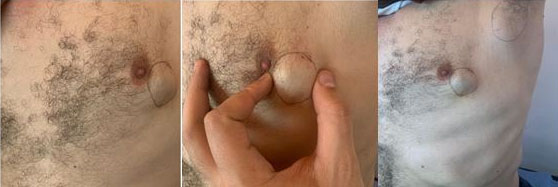
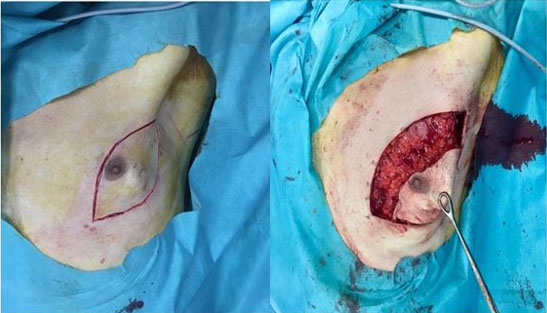
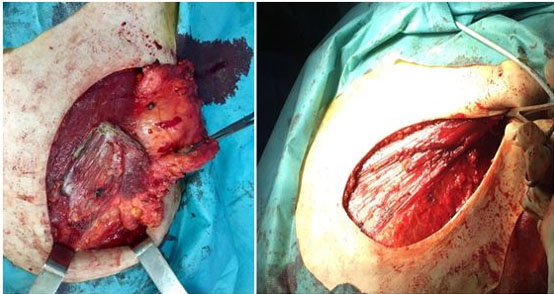
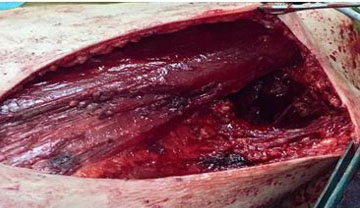
The patient was a 57-year-old man, married with three children, with a history of a mother being followed for breast cancer. He consulted for a self-palpation of a left breast mass evolving for three months. On clinical examination, a node of 3.5 cm was noted in the left external quadrant, without inflammatory signs or nipple discharge, associated with two left axillary lymphadenopathy (Figure 1). Breast ultrasound revealed lobulated, hypoechogenic, and homogeneous vascularized formation with color Doppler, in contact with the large pectoral muscle, measuring 25 mm × 25 mm × 17.5 mm (Figure 2). The left axillary nodes had lobulated contours, and were hypoechogenic and heterogeneous, with some having a central hilum loss and measuring 2 × 4 cm, 2 × 2.6 cm, and 1.6 × 3 cm respectively. This examination was classified BIRADS 4 according to the ACR. A tru-cut biopsy confirmed the presence of a non-specific invasive breast carcinoma of grade II SBR, with a negative molecular phenotype B HER2. Axillary cytopuncture is negative. Subsequently, the patient underwent a left total mastectomy breast tissue and involved skin was removed with axillary dissection. Pectoralis fascia was usually preserved; however, due to adhesion of the lymph node, it was sectioned (Figure 3 and Figure 4) and the axillary nodes were removed through the same incision following the removal of the breast mound (Figure 5). Generally, patients are left with two drains in situ.
DISCUSSION
Breast cancer in men is a rare occurrence, accounting for less than 1% of all cancer cases in men [1],[4]. Diagnosis is generally made at an average age between 59 and 66 years, about a decade later than in women [1],[12].
The most frequent clinical presentation is characterized by a retroareolar hard nodule, often accompanied by clinical cutaneous manifestations, although cutaneous histological alterations are less frequent [13].
Infiltrating ductal carcinoma predominates histologically, accounting for about 80% of cases in various studies [12],[14],[15]. Intra-canal carcinoma occupies the second position, while lobular carcinoma is virtually absent in humans, due to the non-development of the mammary lobules normally observed in the male breast. However, a few cases have been reported in the context of Klinefelter syndrome [15].
The etiology of male breast cancer remains largely enigmatic. Nevertheless, some risk factors seem to be associated with this pathology. Family history of breast cancer [12],[14],[15],[16],[17],[18] as well as links to the genetic mutation BCRA2, was suggested [19],[20]. The presence of gynecomastia, as well as the use of drugs that may cause it, such as cimetidine, ranitidine, captopril, digoxin, verapamil or tricyclic antidepressants, has also been associated with breast cancer in humans. However, the exact nature of these causal relationships remains to be elucidated [12],[14],[16].
Hormonal imbalance, such as real or relative hyperestrogenism, as well as abnormalities in endogenous steroid metabolism and excretion, appears to influence the development of breast cancer in humans [12],[14],[15],[21] , especially in cases of liver dysfunction such as malnutrition, cirrhosis, and kidney failure. In addition, the impact of alcohol consumption is also suspected, as a study found regular alcohol consumption in 55% of breast cancer patients [16]. Another study reported excessive alcohol consumption in 9.6% of their patients, although the exact amount consumed was not specified [21].
The diagnosis of breast cancer in men is usually at more advanced stages, and tumors tend to be larger than those seen in women [22],[23],[24]. The tumor size in men often exceeds 2 cm, with an average of 2.4 cm, while in women, the average is about 2.2 cm [23]. Mammography and ultrasound are used with the same malignancy criteria as in women. However, screening for breast cancer in men is not an option. In a retrospective study conducted from 2001 to 2004 by Hines and colleagues, only 4% of the results were considered suspect, of which only 1% was confirmed as post-biopsy cancer cases.
Breast cancer in men is distinguished by its histological type which differs from that observed in women. Unlike women, where lobular carcinoma is the second most common histological type after infiltrating ductal carcinoma, it is rare in men [8],[9],[25]. According to the American database of Surveillance, Epidemiology, and End Results (SEER), 93.7% of male breast cancers are infiltrating ductal carcinomas, 2.6% are papillary carcinomas, 1.8% are mucinous carcinomas, and only 1.5% are lobular carcinomas. Breast tumors in men are characterized by a strong hormonal propenepedance, although overexpression of HER2 is less common than in women [25],[26].
Many studies have shown that adjuvant hormone therapy improves survival prognosis and complete remission rate in male breast cancer patients. Therefore, many experts recommend routine hormone therapy for all N1 stages and cases of metastasis [12],[21],[27],[28].
The therapeutic management of male breast cancer differs from that observed in women. In women with invasive carcinoma lesion, treatment is primarily conservative or radical surgery in healthy margins, with sentinel ganglion analysis where possible [10],[11]. In contrast, unlike female breast cancer, mastectomy associated with homolateral axillary dissection remains the standard surgical treatment in men [11]. Due to a high incidence of axillary lymph node involvement in men, exploration of the axillary trough is considered necessary in the locoregional management of male breast cancer. Approximately 50–60% of male patients have axillary lymph node involvement [23]. A study by Boughey and colleagues of a population of 30 male breast cancer cases and 2,784 female breast cancer cases found that the sentinel ganglion detection rate was 100% in men and 98.3% in women. When the sentinel ganglion is positive, complementary axillary dissection has a more massive invasion in men than in women, with proportions of 62.5% in men and 20.7% in women [29], respectively. However, these results are based on a limited number of cases due to the recent development of this procedure and the rarity of male breast cancer. Larger scale feasibility studies would be needed to validate this approach in men.
Adjuvant radiotherapy remains a controversial topic, as studies have not shown any benefit in terms of overall survival. However, it reduces the risk of local recurrence, with a 5-year rate ranging from 3% to 20% [30]. Similarly, the use of chemotherapy remains discussed in the literature. It is considered beneficial in younger patients with lymph node involvement or in those considered at high risk, characterized by poor prognostic factors such as the absence of hormonal receptors [31].
In contrast, hormone therapy is of major importance in the adjuvant treatment of male breast cancer due to the high frequency of hormone receptor positivity [21],[22].
Breast cancer in men seems to have a worse prognosis than in women, which is attributable to a later detection, leading to a more advanced stage of the disease in men. However, when a comparison is made between men and women with identical stage and grade tumors, no difference is observed in terms of complete remission rate and survival [32].
A worrying observation from many studies is the prolonged delay between the onset of the first symptoms and medical consultation, with an average of 6–22 months depending on the series. It seems that men consult more quickly for gynecomastia problems than for the appearance of a nodule [12],[13].
Very early forms of breast cancer are often detected in women thanks to the implementation of systematic screening in industrialized countries. Overall five-year survival varies across studies, ranging from 43% to 79%, while at 10 years it ranges from 10% to 56% [21],[22],[23],[24].
CONCLUSION
Most cases of male breast cancer are sporadic, but genetic risk factors may also play a role. Clinically, it has similarities to that observed in women, including more frequent hormonal dependence. However, the overall prognosis is currently less favorable in men, partly due to the frequent presence of comorbidities and the occurrence of second cancers.
REFERENCES
1.
2.
Zehr KR. Diagnosis and treatment of breast cancer in men. Radiol Technol 2019;91(1):51M–61M.
[Pubmed]

3.
Yetkin G, Celayir MF, Tanik C, Citgez B, Uludag M, Mihmanli M. Male breast cancer: A 10 year retrospective case series in a tertiary care hospital. J Pak Med Assoc 2019;69(8):1209–12.
[Pubmed]

4.
Sedighi A, Hamed EA, Mohammadian K, Behnood S, Kalaghchi B. Clinicopathologic characteristics of male breast cancer: A report of 21 cases in radiotherapy center of Hamedan, Iran. Asian Pac J Cancer Prev 2013;14(12):7381–3. [CrossRef]
[Pubmed]

5.
Tischkowitz MD, Hodgson SV, Fentiman IS. 19. Male breast cancer: Aetiology, genetics and clinical management. Int J Clin Pract 2002;56(10):750–4.
[Pubmed]

6.
Reis LO, Dias FG, Castro MA, Ferreira U. Male breast cancer. Aging Male 2011;14(2):99–109. [CrossRef]
[Pubmed]

7.
Ottini L, Palli D, Rizzo S, Federico M, Bazan V, Russo A. Male breast cancer. Crit Rev Oncol Hematol 2010;73(2):141–55. [CrossRef]
[Pubmed]

8.
Giordano SH. A review of the diagnosis and management of male breast cancer. Oncologist 2005;10(7):471–9. [CrossRef]
[Pubmed]

9.
Rohini B, Singh PA, Vatsala M, Vishal D, Mitali S, Nishant S. Pleomorphic lobular carcinoma in a male breast: A rare occurrence. Patholog Res Int 2010;2010:871369. [CrossRef]
[Pubmed]

10.
Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2013. Ann Oncol 2013;24(9):2206–23. [CrossRef]
[Pubmed]

11.
Cloyd JM, Hernandez-Boussard T, Wapnir IL. Outcomes of partial mastectomy in male breast cancer patients: Analysis of SEER, 1983–2009. Ann Surg Oncol 2013;20(5):1545–50. [CrossRef]
[Pubmed]

12.
el Hajjam M, Khaiz D, Benider A, et al. Cancer of the breast in men. Apropos of 50 cases. [Article in French]. J Chir (Paris) 1995;132(3):131–6.
[Pubmed]

13.
Ratón JA, Bilbao I, Gardeazábal J, et al. Skin involvement in male breast carcinoma. Arch Dermatol 1998;134(4):517–8. [CrossRef]
[Pubmed]

14.
Memon MA, Donohue JH. Male breast cancer. Br J Surg 1997;84(4):433–5. [CrossRef]
[Pubmed]

15.
Jaiyesimi IA, Buzdar AU, Sahin AA, Ross MA. Carcinoma of the male breast. Ann Intern Med 1992;117(9):771–7. [CrossRef]
[Pubmed]

16.
O’Hanlon DM, Kent P, Kerin MJ, Given HF. Unilateral breast masses in men over 40: A diagnostic dilemma. Am J Surg 1995;170(1):24–6. [CrossRef]
[Pubmed]

18.
Lynch HT, Lynch J, Conway T, et al. Hereditary breast cancer and family cancer syndromes. World J Surg 1994;18(1):21–31. [CrossRef]
[Pubmed]

19.
Hill A, Yagmur Y, Tran KN, Bolton JS, Robson M, Borgen PI. Localized male breast carcinoma and family history. An analysis of 142 patients. Cancer 1999;86(5):821–5. [CrossRef]
[Pubmed]

20.
Storm HH, Olsen J. Risk of breast cancer in offspring of male breast-cancer patients. Lancet 1999;353(9148):209. [CrossRef]
[Pubmed]

21.
Goss PE, Reid C, Pintilie M, Lim R, Miller N. Male breast carcinoma: A review of 229 patients who presented to the Princess Margaret Hospital during 40 years: 1955–1996. Cancer 1999;85(3):629–39. [CrossRef]
[Pubmed]

22.
Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: A population-based study. Cancer 2004;101(1):51–7. [CrossRef]
[Pubmed]

23.
Korde LA, Zujewski JA, Kamin L, et al. Multidisciplinary meeting on male breast cancer: Summary and research recommendations. J Clin Oncol 2010;28(12):2114–22. [CrossRef]
[Pubmed]

24.
Borgen PI, Wong GY, Vlamis V, et al. Current management of male breast cancer. A review of 104 cases. Ann Surg 1992;215(5):451–7 [CrossRef]
[Pubmed]

25.
Anderson WF, Devesa SS. In situ male breast carcinoma in the surveillance, epidemiology, and end results database of the National Cancer Institute. Cancer 2005;104(8):1733–41. [CrossRef]
[Pubmed]

26.
Rayson D, Erlichman C, Suman VJ, et al. Molecular markers in male breast carcinoma. Cancer 1998;83(9):1947–55. [CrossRef]
[Pubmed]

27.
Williams WL Jr, Powers M, Wagman LD. Cancer of the male breast: A review. J Natl Med Assoc 1996;88(7):439–43.
[Pubmed]

28.
Clark JL, Nguyen PL, Jaszcz WB, Jatoi A, Niehans GA. Prognostic variables in male breast cancer. Am Surg 2000;66(5):502–11.
[Pubmed]

29.
Boughey JC, Bedrosian I, Meric-Bernstam F, et al. Comparative analysis of sentinel lymph node operation in male and female breast cancer patients. J Am Coll Surg 2006;203(4):475–80. [CrossRef]
[Pubmed]

30.
Lenfant-Pejovic MH, Mlika-Cabanne N, Bouchardy C, Auquier A. Risk factors for male breast cancer: A Franco-Swiss case-control study. Int J Cancer 1990;45(4):661–5. [CrossRef]
[Pubmed]

31.
Cutuli B, Le-Nir CCS, Serin D, et al. Male breast cancer. Evolution of treatment and prognostic factors. Analysis of 489 cases. Crit Rev Oncol Hematol 2010;73(3):246–54. [CrossRef]
[Pubmed]

32.
Willsher PC, Leach IH, Ellis IO, Bourke JB, Blamey RW, Robertson JF. A comparison outcome of male breast cancer with female breast cancer. Am J Surg 1997;173(3):185–8. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Amal Cherkaoui - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Meriem Nouri - Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Sara Tossi - Conception of the work, Design of the work, Acquisition of data, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Hasna Armel - Conception of the work, Design of the work, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Younes Bencherifi - Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Mustapha Benhassou - Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Simohamed Ennachit - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Mohamed El Karroumi - Conception of the work, Design of the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2024 Amal Cherkaoui et al.. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.