|
Case Report
Micropapillary carcinoma of the breast: A case report and review of the literature
1 Gynaecology Department, Ibn Rochd University Hospital, Faculty of Medicine and Pharmacy, Hassan II University, Casablanca, Morocco
Address correspondence to:
Sabiri Rachida
Gynaecology Department, Ibn Rochd University Hospital, Faculty of Medicine and Pharmacy, Hassan II University, Casablanca,
Morocco
Message to Corresponding Author
Article ID: 100037G06SR2024
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Rachida S, Cherkaoui A, Benhaddougua K, Zine M, Houssine B, Sakher M, Naima S. Micropapillary carcinoma of the breast: A case report and review of the literature. Edorium J Gynecol Obstet 2024;9(2):1– 4.ABSTRACT
Introduction: A rare entity accounting for less than 3% of all breast carcinomas. Its prognosis is poor due to the presence of vascular emboli and lymph node metastases. However, the micropapillary appearance is not an independent prognostic factor.
Case Report: We report the case of a 38-year-old female patient who presented with a mass of the left breast, the anatomopathology of which was in favor of an invasive micropapillary carcinoma (IMPC). The clinical and radiological presentations are characteristically non-specific: nests of tumor cells with irregular peripheral borders separated from the fibrocollagenous stroma by a clear space. There is no conjunctivo-vascular axis in the middle of the tumor cells. The mitotic index is generally high.
Conclusion: This type of carcinoma is characterised by an aggressive evolution and unfavourable prognosis, frequent alterations of chromosome 8 and a luminal B phenotype, and histologically.
Keywords: Breast, Micropapillary carcinoma, Rare entity
INTRODUCTION
Invasive micropapillary carcinoma (IMPC) of the breast is defined histologically by the presence of small clusters and nests of tumor cells arranged within well-demarcated clear spaces resembling lymphatic vessels. It is a rare histological variant of breast cancer.
Invasive micropapillary carcinoma was first described by Siriangkul et al. in 1993 [1]. This entity is classified by the World Health Organisation (WHO). It accounts for less than 3% of all breast carcinomas and has a poor prognosis due to the presence of vascular emboli and lymph node metastases. Based on an observation and a review of the literature, we discuss the main characteristics of invasive micropapillary carcinoma (IMPC).
CASE REPORT
A 38-year-old woman, with no notable medical history, began experiencing symptoms nine months ago when she noticed a 10 cm mass in her left breast accompanied by skin retraction and enlarged lymph nodes on the same side. There were no apparent abnormalities in the contralateral breast. A follow-up ultrasound scan of the left breast indicated an additional opacity in the upper outer quadrant, characterized by poorly defined oval shape, irregular borders, and heterogeneous hypoechoic structure with calcifications. This formation measured 45.3 × 42.6 mm and showed disruption of the glandular tissue. No abnormalities were found in the right breast or axillary lymph node areas. A biopsy was carried out on the tumor, revealing an invasive ductal carcinoma (IDC) with a micropapillary SBRIII component with carcinomatous emboli, central necrosis RH 100%; HER2 (–); Ki67:80%. The patient therefore received 4 courses of CMT:paclitaxel and C4 of AC 60 (Doxorubicin and Cyclophosphamide) before being referred to us for surgery.
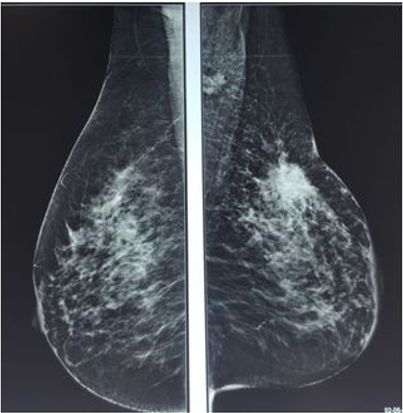
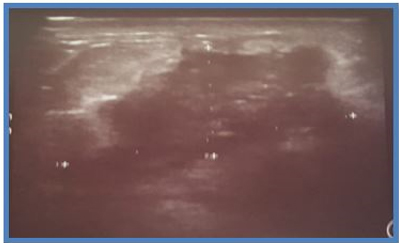
On post-chemotherapy clinical examination: a mass at the junction of the SQs of the left breast measuring 3.5 × 4 cm, mobile in relation to the two cutaneous and deep planes. On ultrasound mammography (Figure 1), there is a dense, spiculated opacity extending across the subcutaneous tissue, accompanied by dusty microcalcifications and evident skin retraction. Additional areas of opacity were observed at the level of the inner quadrants, along with thickening of the skin covering the posterior areolar margin. The ultrasound complement (Figure 2) revealed a large, poorly defined oval formation with irregular, spiculated borders and heterogeneous hypoechoic structure, containing calcifications and surrounded by fatty infiltration. This formation measured 21 × 30 mm. Other nodules were present in the lower outer quadrant (LOQ) and lower inner quadrant (LIQ) measuring 13 and 7 mm, in addition to thickening of the skin covering, hypoechoic axillary adenopathy (ADP) with irregular contours measuring 12 mm. The patient underwent a mastectomy with homolateral lymph node dissection and the final pathological examination showed a residual invasive micropapillary carcinoma, grade SBR II, measuring 3 × 3. 2 cm, no ductal component in situ, base of nipple infiltrated, no Paget’s disease of the nipple, angio-lymphatic invasion, nearest deep border: 1.3 cm, with immunohistochemistry estrogen receptor (RE): 5%, progesterone receptor (RP): 70%, Ki67: <5%, HER2: in favor of a Luminal B profile. Left axillary lymph node dissection revealed 3 out of 12 lymph nodes to be positive for cancer. The patient was then referred to the oncology center.
DISCUSSION
Micropapillary carcinomas are one of the rarest subtypes of cancer, representing 3% of breast cancers, and were first identified by Siriaunkgul and Luna-Moré [1],[2].
From a clinical and evolutionary point of view, multivariate analyses have scarcely shown that this subtype in any way constitutes an independent factor impacting on survival, compared with infiltrating ductal carcinomas of the same stage [2],[3]. Nevertheless, the majority of series, even those with few patients overall, report an increased rate of local recurrence.
Otherwise, it is imperative to emphasize that the micropapillary subtype can be found in other locations such as the lung, bladder, and salivary glands. In these organs, a similar morphology may be a poor prognostic factor [4].
Histologically, the findings are characterized by clusters of cells, sometimes exhibiting a central cavity and a distinct peripheral space. It is found in two forms of proliferation, the pure form or in composite form, associated with another histological type which is most often ductal. Whether pure or mixed, micropapillary carcinoma is characterized by frequent lymphatic dissemination [5]. This involvement can be observed individually depending on its size.
In terms of immunohistochemistry, all the studies involved a small number of patients, with the smallest involving nine and the largest 83. In two studies published in 2008 and 2009, Reis-Filho’s team showed that micropapillary carcinomas were divided into a luminal phenotype (expression of hormone receptors without HER2 overexpression) in around 90% of cases and a HER2 phenotype in around 10% of cases (with HER2 overexpression and/or amplification) [6],[7]. These two publications showed that tumors with a luminal phenotype were often accompanied by an increased proliferative index, leading them to be classified as luminal B, which is the case for our patient.
Molecular level: According to the literature, the overall number of chromosomal abnormalities is slightly lower than that seen in ICCs, but higher than that seen in tubular carcinomas. The most remarkable finding concerns chromosome 8, where there is a loss of the short arm and gains of the long arm in 100% and 88% of micropapillary carcinomas respectively. In addition, in 63% of these tumors there is a loss of the p21-pter part associated with a gain in 8p11-p12. This transition between gains in 8p11-p12 and losses in p21-pter is associated with breakpoints, most often associated with chromosomal translocations [8]. Two foci of recurrent breakpoints have been identified, the first at the telomeric end of the FGFR1 amplification and the second more distal located in the NRG1/Neuregulin gene, which codes for the ligand of the ERBB3 and ERBB4 receptors [9]. In addition to alterations in chromosome 8, loss of the short arm of chromosome 17 associated with gains in 17q is frequently found in micropapillary carcinomas. One study initially suggested that overexpression of the HER2 protein linked to amplification of the ERBB2 gene, located at 17q12, could be more frequent in more aggressive micropapillary histological subtypes [10]. The paucity of studies characterizing the genomic alterations in these tumors shows us that chromosomes 8 and 17 are the most frequently altered in micropapillary carcinomas. At transcriptomic level, a single study of 8 cases showed that the expression profile was consistent with a homogeneous group of carcinomas expressing hormone receptors [11].
The prognosis for invasive breast carcinoma (IBC) is unfavorable due to its lymphophilicity and its high proportion of vascular emboli. Carcinomatous emboli are seen in more than 60% of cases. Lymph node metastases occur in 95% of cases, and the number of lymph nodes affected is greater than in the classic ductal variant.
The recent and limited knowledge of this rare entity does not allow, until now, any change in the therapeutic protocol. The sentinel lymph node method is discouraged because of the high risk of lymph node metastasis. Similarly, the high risk of local recurrence calls for rigorous and meticulous monitoring of these patients.
CONCLUSION
The morphological, genomic, and transcriptional characteristics of micropapillary carcinomas make them an entity in their own right. In addition to its suggestive morphology, this type of carcinoma is characterized by an aggressive evolution and unfavorable prognosis, frequent alterations of chromosome 8 and a luminal B phenotype.
REFERENCES
1.
Siriaunkgul S, Tavassoli FA. Invasive micropapillary carcinoma of the breast. Mod Pathol 1993;6(6):660–2.
[Pubmed]

2.
Luna-Moré S, Gonzalez B, Acedo C, Rodrigo I, Luna C. Invasive micropapillary carcinoma of the breast. A new special type of invasive mammary carcinoma. Pathol Res Pract 1994;190(7):668–74. [CrossRef]
[Pubmed]

3.
Nassar H, Wallis T, Andea A, Dey J, Adsay V, Visscher D. Clinicopathologic analysis of invasive micropapillary differentiation in breast carcinoma. Mod Pathol 2001;14(9):836–41. [CrossRef]
[Pubmed]

4.
Nassar H. Carcinomas with micropapillary morphology: Clinical significance and current concepts. Adv Anat Pathol 2004;11(6):297–303. [CrossRef]
[Pubmed]

5.
Paterakos M, Watkin WG, Edgerton SM, Moore DH 2nd, Thor AD. Invasive micropapillary carcinoma of the breast: A prognostic study. Hum Pathol 1999;30(12):1459–63. [CrossRef]
[Pubmed]

6.
Marchiò C, Iravani M, Natrajan R, et al. Genomic and immunophenotypical characterization of pure micropapillary carcinomas of the breast. J Pathol 2008;215(4):398–410. [CrossRef]
[Pubmed]

7.
Marchiò C, Iravani M, Natrajan R, et al. Mixed micropapillary-ductal carcinomas of the breast: A genomic and immunohistochemical analysis of morphologically distinct components. J Pathol 2009;218(3):301–15. [CrossRef]
[Pubmed]

8.
Gelsi-Boyer V, Orsetti B, Cervera N, et al. Comprehensive profiling of 8p11-12 amplification in breast cancer. Mol Cancer Res 2005;3(12):655–67. [CrossRef]
[Pubmed]

9.
Adélaïde J, Huang HE, Murati A, et al. A recurrent chromosome translocation breakpoint in breast and pancreatic cancer cell lines targets the neuregulin/NRG1 gene. Genes Chromosomes Cancer 2003;37(4):333–45. [CrossRef]
[Pubmed]

10.
Varga Z, Zhao J, Ohlschlegel C, Odermatt B, Heitz PU. Preferential HER-2/neu overexpression and/or amplification in aggressive histological subtypes of invasive breast cancer. Histopathology 2004;44(4):332–8. [CrossRef]
[Pubmed]

11.
Weigelt B, Horlings HM, Kreike B, et al. Refinement of breast cancer classification by molecular characterization of histological special types. J Pathol 2008;216(2):141–50. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Sabiri Rachida - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
A Cherkaoui - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
K Benhaddougua - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
M Zine - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Boufettal Houssine - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Mahdaoui Sakher - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Samouh Naima - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2024 Sabiri Rachida et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.